Abstract
Objective: Myelodysplastic Syndrome (MDS) is a special myeloid neoplasm with still limited treatment choices and poor survival. Although current therapies including HMAs have been shown to prolong survival, only 40-50% of patients respond to therapy, and responses are universally transient, with loss of response within 2 years. With the increasing understanding of the pathogenesis of MDS in recent years, MDS was found closely associated with the bone marrow microenvironment, including immune microenvironment (IME). More and more evidences show that the immune microenvironment of MDS is related with its disease characteristics and prognosis. Knowing more of the IME of MDS may facilitate the diagnose and treatment on MDS. However, till now little was known about the bone marrow immune microenvironment of MDS. Previous studies mostly applied multiparameter flow cytometry and based on the PB samples or frozen bone marrow fluid samples, less was known about the immune landscape of MDS bone marrow biopsies.
Methods: We use multispectral imaging of multiplex immunofluorescence (mIF) to analyze the bone marrow biopsy slides and explore the bone marrow immune microenvironment of MDS patients. A total of 20 de novo MDS patients, 20 post-treatment MDS patients and 20 normal controls were included in this study. In HMAs treated group, 10 patients were response to HMA and another 10 patients were not response. HMAs were either decitabine with a dose at 20mg/m2 d1-5 or 5-Azacytidine with a dose at 75mg/m2 d1-7. The multiplex staining panel could differentiate the distinct cell subpopulations into CD34+CD117+ cells, CD8+ cells, PD1+ cells, CTLA4+ cells, CD8+PD1+ cells and CD8+CTLA4+ cells, CD8-PD1+ cells and CD8-CTLA4+ cells, respectively. We analyzed the proportion of these cells in MDS patients and normal controls, and analyzed the proportion of these cells in MDS patients with different IPSS risk stratifications, and we also assessed the degree of myelofibrosis in MDS patients and analyzed the relationship between myelofibrosis and immune cells.
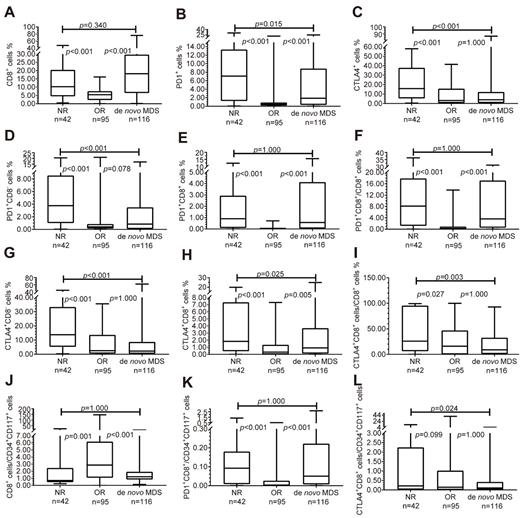
Results: We found that compared with the normal control, the proportion of CD8+ cells in de novo MDS patients increased, and CD8+PD1+ T cells increased too, but the CD8+CTLA4+ T cells decreased. Compared with the lower-risk groups, CD8+ T cells as well as CD8+PD1+ T cells increased in higher-risk groups, while CD8+CTLA4+ T cells decreased. We also found a higher CD8+ T cells as well as CD8+/PD1+ T cells in MDS-F patients, especially in MDS-F patients with higher IPSS risk levels. We also found that compared with the normal control, the proportion of CD8+ T cells per CD34+CD117+ cell reduced, and was lower in IPSS int-2 risk/high-risk group than that in low-risk/int-1 risk group. But the proportion of CD8+PD1+/CD34+CD117+ increased compared with normal control. To analyze the bone marrow immune microenvironment after HMAs treatment, CD8+PD1+ T cells decreased in patients who are effective to HMAs, but still remain high in patients who had no response to HMAs, so did the proportion of CD8+PD1+/CD8+ and CD8+PD1+/CD34+CD117+. However, such findings were not found on CD8+/CTLA4+ T cells.
Conclusions: Our research findings provide a new insight on the bone marrow IME of MDS. The results confirmed that the immune checkpoint molecule PD-1 positive CD8+ T cells are abundant in MDS IME and associated with the risk. However, CTLA4 may not be a major molecule interfere with the IME of MDS. Our result also suggest that PD-1 inhibitor combines with HMAs might be used as a first-line therapy for de novo MDS patients to cover those patients who may not have response to HMAs. Also, PD-1 inhibitor may cover higher risk MDS and MDS-F, the latter is a high-risk subtype of MDS.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal